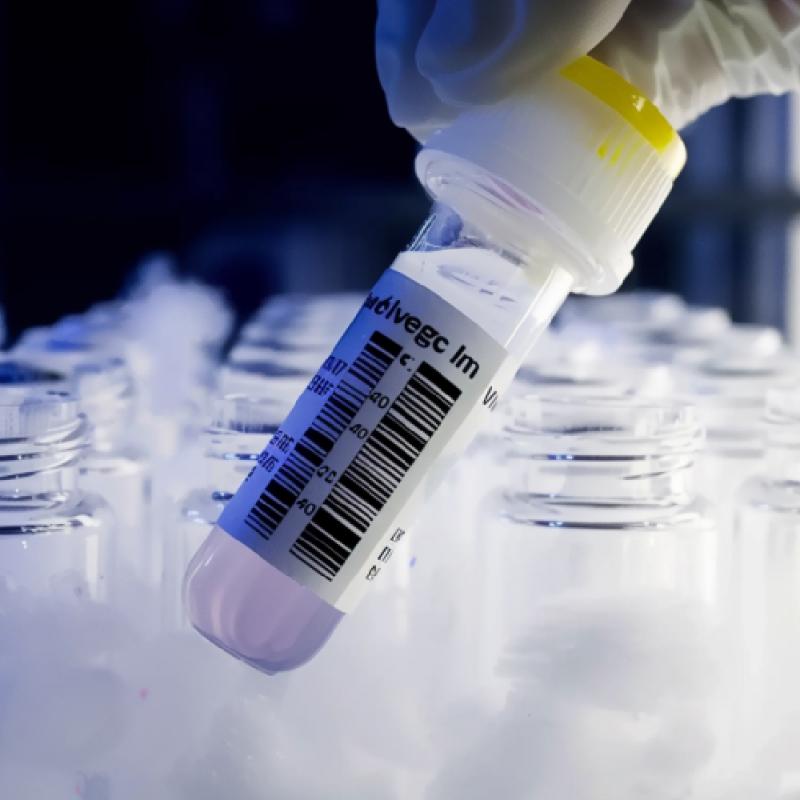
Cold Storage Temperature Labels Reduce Pharma Cold-Chain Waste
By hqt
Cold Storage Temperature Labels are simple visual indicators designed for controlled cold environments. They show status at a glance and survive frost, condensation, and routine cleaning. In pharma, they protect vaccines, biologics, and temperature-sensitive APIs. Labs use them for specimens, cryo vials, and biobank tubes. Logistics teams rely on them in depots, reefer trucks, and last-mile fridges. Hospitals and clinics use them for safe receiving and storage. Cell and gene workflows demand cryogenic durability. But how do these labels actually cut waste – and why do they outperform costly gadgets? Keep reading to see where the savings come from and what most teams overlook.

Why Cold Storage Temperature Labels Matter In Pharma
When temperature control slips, losses climb fast. A few degrees off can mean scrapped vaccines, delayed studies, and hours of QA investigations. Most excursions don’t happen in the lab; they happen in the messy real world – on a loading dock, during a hand-off, or in a clinic fridge that’s been opened all morning. Paper notes and ad-hoc stickers are easy to misread. Data loggers help, but they’re hard to scale to every unit and every box.
At Crystal Code, we design Cold Storage Temperature Labels to make status obvious at a glance. If a sample is safe, teams move. If it’s questionable, they act. That clarity reduces handling errors, speeds disposition, and keeps product moving. The result is lower write-offs and fewer bottlenecks across QA, logistics, and regulatory workflows.
- The Real Cost Of Excursions
Waste is more than the vial you toss. Every excursion triggers CAPA work, quarantine space, re-labeling, re-testing, and coordination with sponsors or health authorities. A label that stays put, stays readable, and survives frost and cleaning prevents those downstream costs. Simple, visible control closes the gap between SOPs and everyday practice.
How Crystal Code Builds Labels For The Cold
Pharma doesn’t live at one temperature. Clinics run standard freezers, depots operate deep-freeze, and cell & gene programs go cryogenic. Cold Storage Temperature Labels must span that entire stack – right down to liquid nitrogen at −196 °C (−320 °F). That’s where Crystal Code’s materials engineering stands apart.
- Engineered For −196 °C Cryogenics
Our Liquid Nitrogen Label line uses cryogenic-grade films and adhesives that perform through the harshest cycles – filling, plunge-freezing, storage, and thaw. The construction prevents cracking, curling, and ink lift when containers frost over or go through rapid temperature shifts. Even after repeated freeze–thaw events, the identifier stays intact and legible, protecting sample identity when it matters most.
• Ultra-low temperature resistance proven in LN₂ environments at −196 °C
• Moisture-proof, chemical-resistant surface that resists frost, condensation, and routine wipes with ethanol
• Readable, smudge-resistant print that holds up to handling and cleaning
- Adhesion That Doesn’t Quit
In cold storage, weak adhesion is the silent failure. A corner lifts, a code mis-scans, and product goes on hold. We tune our cryo-adhesive to grip the real substrates you use – glass, plastics, metal, cryo vials, storage boxes, and tubes. Bonding is immediate and robust, even on chilled surfaces, so labels don’t drift or peel when humidity spikes or containers move from cold rooms to ambient conditions.
- Print Legibility And Compliance
A label that survives the cold but won’t scan still creates waste. Our matte faces provide strong ink receptivity for crisp human-readable text, 2D barcodes, and lot/expiry blocks. Print remains high-contrast after wipe-downs and glove handling. Materials meet SGS, RoHS, and ISO expectations to support quality systems and audit trails in regulated pharma environments.
Simple Deployment, Measurable Results
Technology should fit the process – not the other way around. That’s why Crystal Code ships Cold Storage Temperature Labels in familiar formats that work with the printers you already own. You can roll out in days, not months, and you can prove impact with basic metrics.

- Fast Rollout Plan
Start focused, prove the gain, then scale across lanes and geographies.
• Map your storage zones: refrigerated, frozen, deep-frozen, and cryogenic.
• Match label grade to the zone (e.g., LN₂-rated labels for cell & gene and biobanking).
• Standardize templates: product, batch, 2D code, storage range, and disposition checkbox.
• Print on demand with common inkjet devices – our sheets fit A4/Letter (8.5″×11″ / 210×297 mm) stations.
• Train for reality: gloved application on cold surfaces, wipe-down practices, and visual “pass/fail” cues.
• Audit at 30 days: mislabels, unreadable codes, holds due to identification issues, and excursion-related write-offs.
- What Good Looks Like?
Teams typically report faster receiving checks, fewer QA holds tied to unreadable IDs, and a clear reduction in write-offs. Visual clarity shortens decision time at the dock and in the clinic. Reliable adhesion reduces re-labeling and scanner jams. Consistent print quality improves traceability from depot to bedside – supporting smoother releases and stronger compliance evidence.
Why Crystal Code
Crystal Code blends practical manufacturing with materials science. We engineer Cold Storage Temperature Labels that behave predictably from standard cold rooms to −196 °C cryogenics. Our coatings shrug off condensation and common chemicals. Our adhesives hold when frost appears. Our faces print cleanly and scan instantly. That combination tackles the pain points that drive cold-chain waste: misidentification, damaged labels, and slow decisions.
We also keep deployment simple. You can integrate our labels into current SOPs and existing printing stations without special hardware or software. With strong ink receptivity, waterproof and chemical-resistant construction, and easy peel / easy cut handling, teams adapt quickly. And because our materials are certified (SGS / RoHS / ISO), they align with the documentation you already maintain for audits and validations.
The Bottom Line
Cold-chain waste isn’t only a temperature problem; it’s a visibility problem. Give operators a durable, readable signal that survives the cold, and they make the right call sooner. That is the quiet power of Cold Storage Temperature Labels – less hesitation, fewer holds, and more product reaching patients on time.
Call To Action
Ready to reduce cold-chain waste with a simple, scalable solution? Talk to Crystal Code for samples, print templates, and a quick fit-for-purpose assessment. We’ll help you match each lane to the right Cold Storage Temperature Labels and start saving product on your very next shipment.
- Agodeo inkjet vinyl sticker paper
- AIVA printable vinyl sticker paper
- best inkjet vinyl sticker paper for outdoor use
- cheap printable vinyl sticker paper for outdoor use
- cold chain temperature indicator
- color-change temperature sticker
- Cricut vinyl sticker paper inkjet
- custom vinyl sticker printing
- DIY sticker paper sheets
- durable waterproof sticker paper for inkjet printers
- electronics temperature sticker label
- glossy inkjet vinyl sticker paper
- hot equipment warning sticker
- industrial temperature indicator label
- inkjet sticker paper for Cricut
- matte inkjet sticker paper
- medical temperature indicator sticker
- multi-point irreversible temperature sticker
- Neato vinyl sticker paper
- one-time temperature indicator label
- orajat 1917 printable vinyl
- peak temperature measurement label
- printable vinyl for car decals
- printable vinyl for laptop stickers
- printable vinyl for scrapbooking
- professional quality inkjet vinyl sheets
- reversible temperature monitor label
- self-adhesive printable vinyl
- shipping temperature indicator label
- Tear-resistant sticker paper
- temperature indicator for food safety
- temperature monitoring sticker for transport
- vinyl sticker paper for labels
- waterproof printable vinyl paper
- where to buy inkjet vinyl sticker paper